Assign an Oxidation Number to Each Element in the Reaction
MnO2 H2C2O4 H2SO4 à MnSO4 CO2 H2O. Thus when looking at the equation look for which oxidation state is becoming more positive.

Answered Assign An Oxidation Number To Each Element In The Reaction Co G 2h2 G Ch3oh G In Brainly Com
Monatomic ions have oxidation numbers equal to their charge.

. The alkaline earth metals group II are always assigned an oxidation number of. Assign an oxidation number to each element in the reaction. Total oxidation needs to equal 0 fluorine and hydrogen.
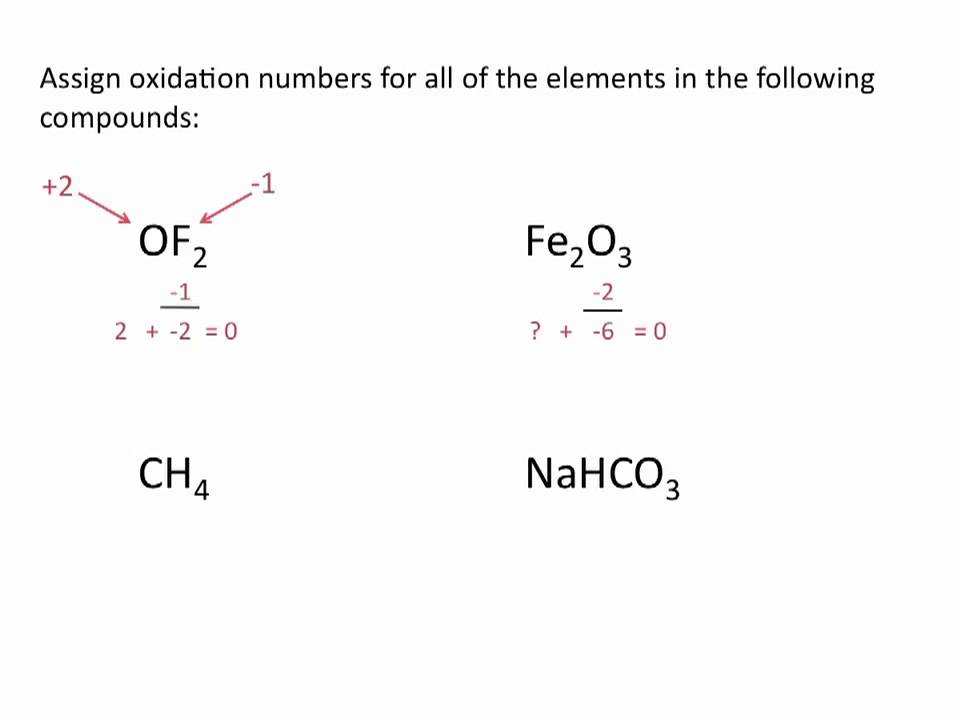
For free elements uncombined state each atom has an oxidation number of zero. Solution for Assign oxidation numbers to each of the elements in the following compounds. These rules make it possible to calculate the oxidation number of an atom in a compound given the oxidation numbers of other atoms in the same compound.
Identify the oxidizing agent and the reducing agent in each redox reaction 1 Chlorine reacts with water to form HCl and oxygen. Superman warworld reading order. CaF2 s H2SO4 aq CaSO4 aq 2HF g - In CaF2 the oxidation number of Ca is 2 and that of F is -1.
The oxidation number of a monatomic ion equals the charge of the ion. I grew up downriver in Allen Park. Answer 1 of 2.
In CH3OH the oxidation number of C is _ that of O is _ and that of H is _. If the reaction is a redox reaction determine what is being oxidized and what is being reduced. There is not a.
Show any calculations you make. Fluorine in compounds is always assigned an oxidation number of -1. State which element is being oxidized and which element is being reduced as well as how you know.
Community high school downtown brooklyn. Determine whether each reaction is a redox reaction. Assign oxidation numbers to each of the elements in the following compounds.
The oxidation number of hydrogen is -1 in compounds containing elements that are less electronegative than hydrogen as in CaH 2. Assign any element that is not combined with any other. In this video well use this method to identify the oxidized and reduced elements in the reaction that occurs between I and MnO₄ in basic solution.
Assign an oxidation number to each element in the reaction. H 2 Br 2 Na Be K O 2 P 4 all have an oxidation number of 0. What are the oxidation numbers for each element in sodium peroxide eqNa_2O_2 eq.
Rules for assigning oxidation numbers. Every element in their a given allotropic form has a property to attract or repel fresh electrons. That is why a unique property called electronegativity has been defined as the tendency of an.
The oxidation number of a monatomic ion equals the charge of the ion. 5 rows A series of rules have been developed to assign oxidation numbers to atoms. Up to 10 cash back Possible Answers.
My education was like most of Michigan people I applied for an in state college. What is the length of. Read yaml file from s3 python.
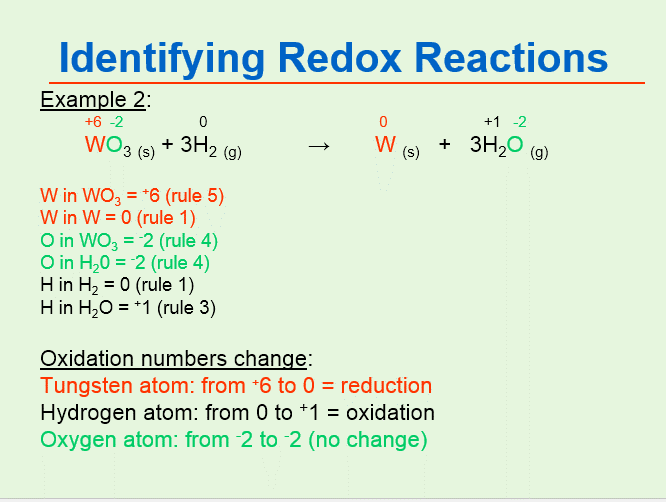
By assigning oxidation numbers to the atoms of each element in a redox equation we can determine which element is oxidized and which element is reduced during the reaction. Alkali metal oxidation numbers 1. The usual oxidation number of hydrogen is 1.
Li 1 Ba 2 2 Fe 3 3 I 1 O 2 2 etc. COg 2H2g CH3OHg In CO the oxidation number of C is _ and that of O is _. An oxidation number is assigned by following a series of rules.
Recall that a reducing agent is being oxidized and is losing electrons. For example the oxidation number of Na is 1. In CH3OH the oxidation state of carbon is -2 that of hydrogen is 1 and that of oxygen is -2.
For example in Uranium hexafluoride UF6 Fluorine has an oxidation number of -1. For the following REDOX reaction state the oxidation number for each element in the reaction. All elements in their a given allotropic form are assigned zero oxidation state.
In H2 the oxidation number of H is _. In CO the oxidation number of carbon is 2 and that of oxygen is -2. Hot n roll gulshan e iqbal block 2 number.
State which element is the oxidizing agent and which element is the reducing agent as well as how you know. Show any calculations you make. In H2 the oxidation number of hydrogen is zero.
The oxidation number of the elements in hyperfluorous acid HFO are. Assign oxidation numbers to each element in the following reactions. M-5 2 and n5 2 are the endpoints of the segment mn on the coordinate plane.
Has an oxidation state of. Start by assigning oxidation states to the elements. The alkali metals group I always have an oxidation number of 1.
Caf2s h2so4aq caso4aq 2hfg Select the correct answer. - In H2SO4 the oxidation number of H is 1 that of S is 6 and that of O is -2. The oxidation number of a free element is always 0.
Assign an oxidation number to each element in the reaction. Andre russell getty images. Working to pay my way through college I worked at a variety of jobs to pay the bills.
The oxidation number of N 3-is -3.
Oxidation Numbers Vce Chemistry

How To Calculate Oxidation Numbers Introduction Youtube
Assigning Oxidation Numbers Chemistry Tutorial Youtube

Oxidation Reduction Reactions 10th Higher Ed Worksheet Scientific Notation Word Problems Chemistry Worksheets Word Problem Worksheets
0 Response to "Assign an Oxidation Number to Each Element in the Reaction"
Post a Comment